Use of AI in Pharmaceutical Industry


The discovery of new drugs, compounds, and biological products requires researchers to process large amounts of data from various sources manually. The workflows are labor-intensive, which results in the high costs of new medicine development.
The implementation of artificial intelligence (AI) in pharmaceuticals helps automate data processing and decision-making. Researchers can rapidly find recent information across vast amounts of studies, run virtual experiments, and conduct automated applicant pre-assessment.
In the article below, you will learn more about the application, regulatory requirements, strategic benefits, and a real-world case study of using AI in the pharma sector.
Applications of ai in pharmaceutical industry
The advanced capabilities of the technology make the diverse use of AI application integrations in the pharmaceutical industry. The key applications of artificial intelligence in pharma are listed below.

1. AI in Drug Discovery
As per the research, the average time-to-market for a new prescription drug ranges from 5.9 to 7.2 years, with over $2.6 billion in spending for commercialization.
The AI’s capability to quickly analyze extensive biological data enables medical researchers to discover new lead compounds and predict their effects quickly. This enables a targeted approach to drug discovery, decreasing the efforts and time needed to discover new drugs through the following methods:
1.1 Predictive modeling
- Problem: AI models trained on pharmaceutical datasets are used for predictive analytics of certain activities without running traditional experimental methods.
- AI solution: Researchers have achieved 82% accuracy in predicting the stability of a certain physical process used for drug discovery. AI model tools give insights into future outcomes, saving time and money.
1.2 Drug repurposing
- Problem: It is a more efficient yet time-consuming drug discovery process because specialists need to manually review the pharmacokinetic profiles of approved drugs.
- AI solution: The utilization of artificial intelligence helps researchers to automatically process large datasets of biomedical chemical data and biological knowledge to find approved drugs that may have additional but undiscovered therapeutic potential. DSP-1181, the first AI-reposed drug, entered clinical trials in 12 months, which was a significant achievement in terms of drug development acceleration with AI.
1.3 Lead optimization
- Problem: Traditional processes are resource-intensive because they need researchers to synthesize and test large numbers of compounds.
- AI solution: AI applications in pharma help analyze the molecular modeling of different elements along with their biological activity with data integrity. It allows researchers to virtually modify the structure-activity relationship (SAR) of molecules to enhance the features of different components.
1.4 De novo drug design
- Problem: In most cases, researchers need to adjust the known compounds and explore libraries for designing new chemical entities.
- AI solution: Using generative AI in pharma, specialists can leverage large amounts of data for the molecular design of entirely new compounds to discover new drug candidates, considering the unique chemical binding requirements specified by researchers.
1.5 Bioactivity and toxicity prediction
- Problem: The determination of safety and efficacy is resource-consuming and raises ethical concerns because of animal testing.
- AI solution: The usage of AI-driven tools helps identify potentially harmful compounds in the early stages by virtual screening the existing academic literature, libraries, and research.
Build an AI tool with custom functionality
Business First
Code Next
Let’s talk
2. Clinical Trials Optimization with AI
Traditional patient triage methods mostly rely on manual data processing. It is a significant bottleneck, considering the lack of skilled professionals and the increasing number of patients in clinical trials.
2.1 Patient triage
- Problem: Statistics say that over 80% of trials exceed the defined enrollment deadlines. Specialists need to manually assess a lot of medical records to pick the best-matching participants for patient recruitment. Moreover, they need to consider a lot of various criteria, which makes the triage process more complex.
- AI solution: AI-powered clinical research with advanced algorithms can swiftly analyze large amounts of complex historical clinical trial data, facilitating the pre-screening process for optimizing trial design. The data AI can analyze include genomic profiles, unique clinical characteristics, doctors’ notes, lab reports, etc.
2.2 Real-time monitoring
- Problem: The lack of workforce, usage of isolated solutions, and insufficient process automation make it difficult for triage specialists to monitor data updates in real time and swiftly respond to new changes.
- AI solution: It enables a more efficient alternative to traditional monitoring with predictive modeling. The technology enables specialists to run continuous real-time monitoring and insights extraction for safety outcomes. Moreover, the real-time analysis helps detect issues quickly to respond to severe changes.
2.3 Personalized medicine
- Problem: Usually, healthcare specialists apply the one-size-fits-all approach when selecting treatment methods and prescribing medications due to a lack of data-backed insights into treatment efficacy.
- AI solution: The deep analysis of genomic profiles, clinical histories, medical images, and other details helps develop innovative therapeutic solutions that are tailored to the specific needs of each patient using supervised learning for patient response prediction.
3. AI in Pharmaceutical R&D
Using AI for pharmaceutical R&D automation enhances performance, cuts expenses, and streamlines the development of new medicine.
3.1 Pharmaceutical product development
- Problem: Repetitive testing, drug delivery system analysis, dosage forms control, and other processes are time-consuming and need researchers to explore a lot of extensive studies for medical reporting.
- AI solution: Having access to relevant studies, an AI-driven lab automation system can identify complex drug-biological interactions, detect defects, and find optimized formulations rapidly, avoiding resource-intensive manual testing. Statistics say that AI specialist platforms and ML in the pharma industry algorithms can predict the physicochemical stability with an 82% accuracy for drug development time reduction.
3.2 Pharmacokinetics and pharmacodynamics (PK/PD) studies
- Problem: The traditional processes of predicting human drug behavior are based on real-life experiments with wet lab support. This approach raises ethical concerns, requires extensive funding, and has limited accuracy.
- AI solution: The ability to run in-silico research and adjust parameters decreases the need for human and animal testing in the initial phases. AI and ML in pharma algorithms help pick the best parameters for compound delivery, drug release, and absorption more efficiently under regulatory frameworks.
3.3 Biologics product development
- Problem: The development of biological product development needs specialists to analyze protein structure and function within the ai market in genomics. Also, they need to assess toxicity by running clinical trials.
- AI solution: The adoption of AI tools acquisition enables researchers to analyze large protein data volumes to design biological products with the demanded features. For example, AlphaFold, the AI technology in cancer diagnosis tool, is capable of predicting protein folding, streamlining the research processes by helping scientists to understand how molecules interact with each other for medical insights.
4. AI in the Pharma Supply Chain
Artificial Intelligence helps enterprises gain complete visibility into operations accuracy, detecting bottlenecks and points of growth in supply chain management. Also, it delivers information about events that are likely to occur in the future, helping businesses adjust their workflows to tackle future challenges with supply chain compliance.
4.1 Predictive maintenance
- Problem: Unplanned downtime caused by equipment failures causes serious disruptions in production schedules and financial losses.
- AI solution: Predictive maintenance powered by AI in the pharma industry provides data quality-backed information about equipment that is likely to fail by analyzing data collected from shop floor sensors for inventory management. Also, it helps understand the root causes of a failure impacting drug efficacy.
4.2 Process optimization
- Problem: Continuous monitoring and supervised testing are required when adjusting workflows in the pharmaceutical production process for quality control processes.
- AI solution: AI and machine learning in pharma 4.0 can analyze complex data comprising a lot of different parameters with a predictive analytics model. It helps predict the future of AI-powered in pharma outcomes of certain changes implemented to workflows for demand forecasting. Actionable insights extracted from the provided datasets help fine-tune the existing processes, achieving maximum efficiency in logistics, stock management, and real-time monitoring.
5. Cross-Functional Applications of AI
Vast AI capabilities make it possible to build advanced tools that are applied in different domains. The foremost cross-functional applications of AI in the pharmaceutical industry are as follows.
5.1 Regulatory compliance and drug safety
- Problem: Traditional methods need skilled specialists to manually analyze data, ensuring compliance with regulatory requirements in medical and regulatory affairs.
- AI solution: Tools that use AI for regulatory compliance can pull information from various sources and analyze it, detecting noncompliance concerns or adverse events automatically. It drastically increases the performance of the teams of researchers because they can dedicate their time to more value-added activities.
5.2 AI in medical devices
- Problem: The health data collected by medical devices needs to be manually assessed by doctors, which consumes a lot of time.
- AI solution: In addition to text records, machine vision algorithms can also analyze X-rays, CT scans, and MRIs, providing help in detecting and diagnosing diseases. The AI-driven pre-screening highlights the foremost problems and issues doctors need to examine first.
5.3 Forecasting epidemics and pandemics
- Problem: Early detection of signs of epidemics is a challenging task because it requires continuous analysis of large amounts of data.
- AI solution: AI & ML in pharma algorithms enable the opportunity to effectively monitor health information pulled from different sources by utilizing resources efficiently. For instance, the MSDII-FFNN model has achieved 90% accuracy in predicting the influenza pandemic.
Requirements for generative ai in pharma
The implementation of generative Artificial Intelligence in the pharmaceutical industry sets new challenges that are related to strict control in AI use cases. Researchers must understand how GenAI-driven transformation systems utilize data and discover the reasoning behind the results delivered.

AI Implementation Concerns
The foremost concerns of using AI in pharma are related to the data utilization and sharing by AI systems in biological systems complexity. Check out the top unsettled problems below.
- Intellectual property rights (IPR)—since large volumes of data are required for AI-driven systems, researchers may use copyrighted materials, which are hard to trace due to lack of data availability. Moreover, no rules define the ownership of the outcomes and insights delivered by AI under regulatory pressure.
- Data privacy—the utilization of SaaS AI solutions and large language models (LLM) accessed through APIs, sets pharmaceutical data security concerns with biases in data. Such solutions can access sensitive information protected by a copyright right and learn from unique insights discovered by a company in a global manufacturing network.
- Result validation—there is a risk of an AI system delivering improper research results, especially when poor-quality data is used to train inactive molecules. Hence, the outcomes of AI should always be approved by top pharmaceutical experts to interpret the results.
- Insufficient process understanding—specialists lack understanding of how AI systems analyze data and discover new compounds due to a lack of transparency. It’s crucial for them to understand the reasoning behind results so they can replicate a process when running manual testing and validation with consideration of ethics.
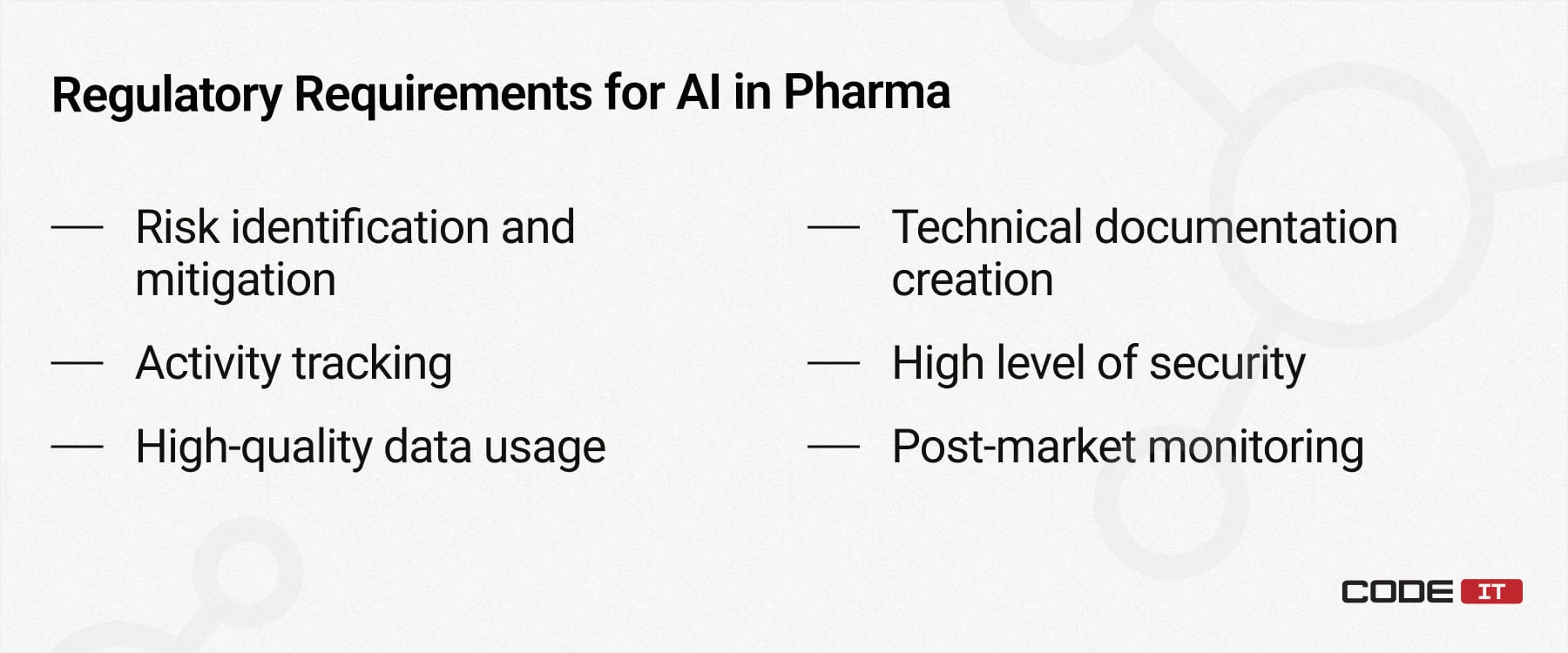
Regulatory Requirements
The European AI Act is the most comprehensive framework that is projected to become a global standard for the fair utilization of artificial intelligence in the future. As per the document, the main requirements are as follows.
- Risk identification and mitigation—pharmaceutical companies have to identify possible risks associated with the usage of AI tools for drug discovery and develop mitigation plans.
- Activity tracking—it’s crucial for researchers to understand how AI makes decisions. Therefore, enterprises in the pharma industry need to implement logging and tracing features to track each step and activity.
- High-quality data usage—relevant and complete datasets need to be used for ML model training to avoid discrimination and improper result delivery.
- Technical documentation creation—the creators of custom AI systems need to maintain detailed documentation describing how a system works. Also, they have to create clear guidelines, ensuring the end user can utilize all the features.
- High level of security—since AI-driven software may process sensitive information, including the personal information of patients, it requires developers to implement strong security measures to avoid data leakage.
- Post-market monitoring—the continuous monitoring after release helps identify emerging issues in the early stages so they can be fixed in new iterations of a solution.
How to build gen AI in pharma
Since there is a lack of all-purpose tools for researchers, companies have to build custom tools with AI capabilities tailored to solving specific tasks. The development of a new application of gen AI in the pharmaceutical industry comprises the following steps:
- Objectives definition
define your business vision and set clear goals for new software development. Map out the problems it should solve and processes to improve. Also, it’s advisable to create a clear description for each feature, along with the definition of done (DoD) parameters.
- AI tools and technologies selection
develop an architecture of a new AI-driven tool and choose the best technologies for throughput. Analyze the pre-built tools, frameworks, libraries, LLMs, and other tools for building your AI software fast.
- Data preparation
collect and prepare data for training ML models. Also, data collection pipelines should be developed to gather new pharmaceutical studies and findings continuously.
- AI tool development
gather a team of software engineers and develop an AI tool with custom features to ensure quality. Validate the accuracy of insights delivered by the developed software and fine-tune it upon a need.
- Monitoring and evaluation
monitor the performance of the new software to ensure that it continuously delivers top-tier results and no parameters need adjustment for compliance.
Use case: self-hosted rag for secure AI in pharma
Under the guidance of our AI department, CodeIT has created a self-hosted LLM and retrieval-augmented generation (RAG) for a company in the pharma sector. Read about the challenges we solved and the unique solutions implemented below.
Problem
Workflows in pharmaceutical companies are labor-intensive because researchers need to explore studies, run experiments, and assess outcomes manually. The search for relevant materials is insufficient because they need to explore directories or use keywords only.
The additional challenges include the risk of error because of a large number of manual operations and overburdened QA teams.
Solution
The tool streamlines unstructured data processing and automates bothersome manual tasks thanks to the following solutions developed:
- Integration with internal repositories for automated data collection
- On-premise AI solutions (self-hosted), eliminating dependency on third-party vendors
- Custom LLM integration (Mistral, Llama, and similar)
- Additional security modules for enhanced secure AI deployment in pharma
The tool ensures top-tier security thanks to the unique measures implemented, including the following:
- Role-based access control (RBAC)
- Real-time logging & monitoring
- Secure API gateways
- Data masking & anonymization
- Regular penetration testing & security audits
- Multi-factor authentication (MFA)
Benefits of Artificial Intelligence in pharmaceutical industry
The implementation of AI-driven tools streamlines operations by enabling data analysis and decision-making. Consequently, researchers and healthcare specialists can get actionable insights and pre-screening results and simulate compound interactions and treatment outcomes.
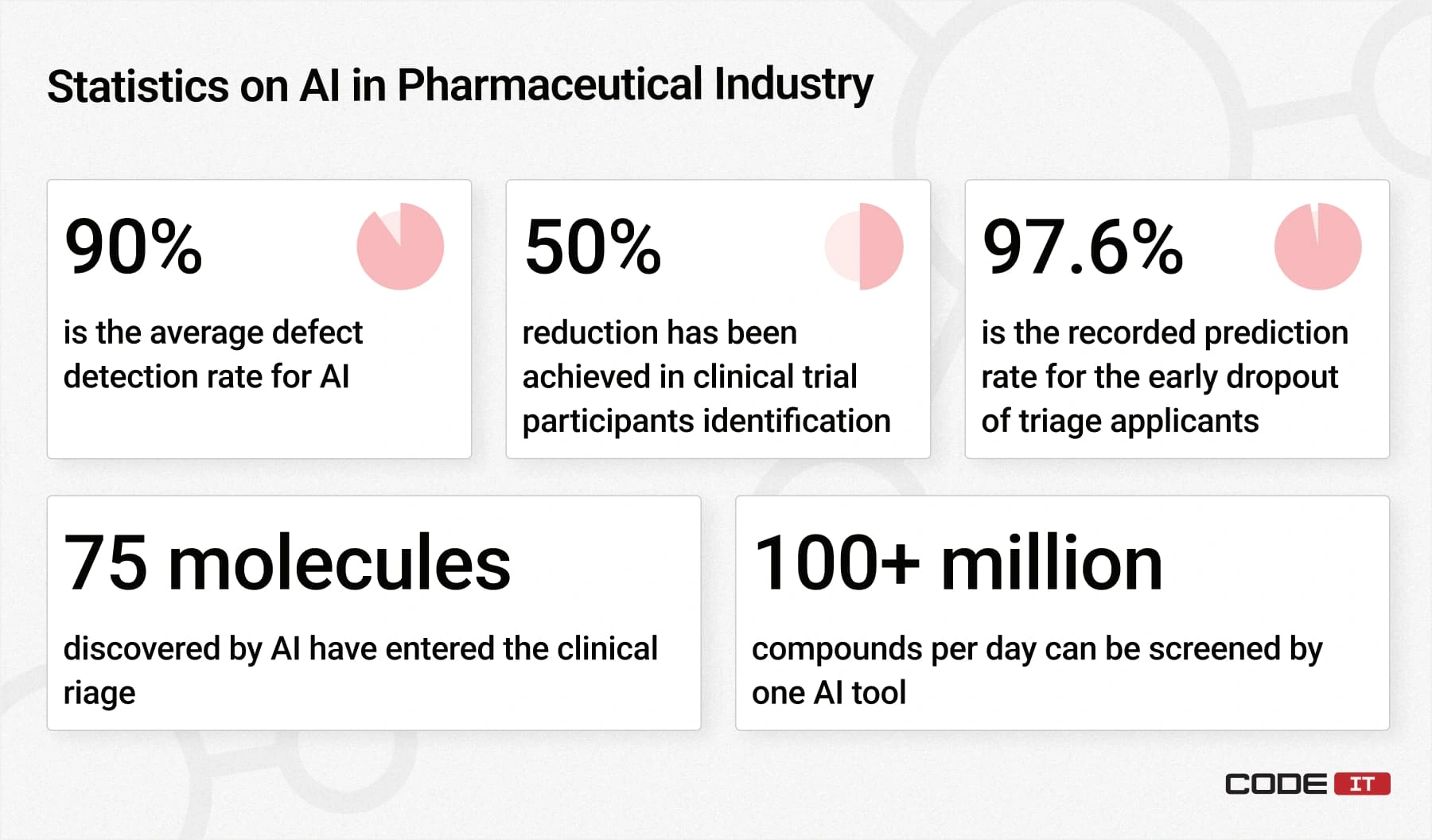
The top measurable achievements of using Artificial Intelligence in the pharmaceutical industry are as follows.
Pharmaceutical success:
- 75 molecules discovered by AI have successfully entered the clinical triage.
- 90% is the average defect detection rate for AI-driven systems, compared to 75% for human workers.
- 50% reduction in the time for identifying clinical trial participants has been achieved with AI systems.
- 100+ million compounds per day can be screened by MIT researchers using a machine-learning algorithm.
- 46 days were spent analyzing 30,000 novel drug-like compounds and extracting the 6 most promising with AI; traditionally, this process could have taken months and millions of US dollars in spending.
- 97.6% is the recorded prediction rate for the early dropout of triage applicants achieved by ML models.
Supply chain management:
- 20% increase in prediction accuracy has been achieved with AI by a pharmaceutical corporation.
- 69% of pharmaceutical companies have implemented AI-driven alerts to monitor their logistics.
- 31% increase in demand forecasting accuracy in the pharmaceutical supply chain has been recorded with machine learning.
Final words
The use of AI in the pharmaceutical industry is mainly described by the development and training of custom ML models. They help swiftly process large volumes of data, extracting actionable insight and developing predictions. Also, they help quickly find relevant information for efficient research, highlighting the important role of AI in pharma digital transformation.
The foremost applications of AI-driven solutions for pharma include the following:
- Drug discovery—AI helps discover new compounds and medicine by analyzing extensive biological data.
- Clinical trial optimization—AI automates data analysis and decision-making. It improves operational performance by running patient pre-assessment and picking the best-matching applicants.
- Pharmaceutical R&D—AI is capable of analyzing a lot of studies and biological information for new pharmaceutical product development.
- Supply chain management—AI-driven supply chain management software helps accurately predict future demand, pick the best transportation routes, detect bottlenecks, and more to enable efficient operations.
- Cross-functional applications—AI can be applied in various domains to tackle industry-specific tasks.
Ready to unlock new opportunities with AI

Business First
Code Next
Let’s talk
FAQ
AI is used to automate data processing and decision-making. Key applications include:
- Drug discovery
- Clinical trial optimization
- Pharmaceutical R&D
- AI in the pharma supply chain
- Cross-functional applications
The future involves the continued development of custom ML models for data analysis and prediction. The European AI Act may become a global standard for fair AI use. Companies are developing custom, secure AI solutions like self-hosted LLMs to enable secured data management.
Key regulatory requirements include:
- Risk identification and mitigation
- Activity tracking
- High-quality data usage
- Technical documentation creation
- High level of security
- Post-market monitoring
Building AI software involves the following steps:
- Objectives definition
- AI tools and technologies selection.
- Data preparation.
- AI tool development
- Monitoring and evaluation
The benefits of using conversational AI in healthcare and pharma include:
- Increased speed and efficiency
- Cost reduction
- Improved accuracy
- Identification of novel candidates
- Enhanced lead optimization
- Reduced animal testing
- Screening large numbers of compounds
Build your ideal
software today